QUALITY & ACCREDITATION AND CONTRIBUTION
Quality Assurance Certification
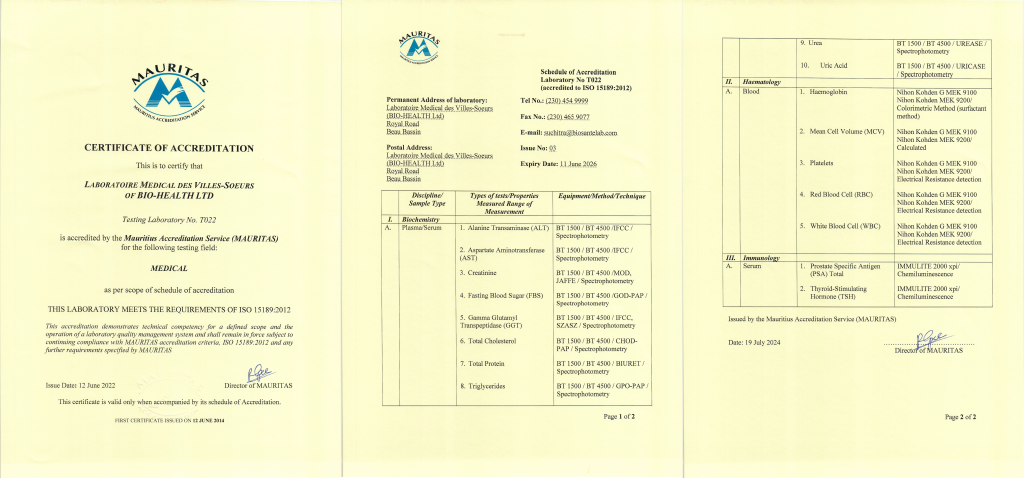
BIOSANTE Medical Laboratories are the FIRST Company in Mauritius to be accredited ISO 15189, in the Medical Testing Field by MAURITAS – the sole national authority in Mauritius providing unified service for the accreditation of calibration and testing laboratories, inspection bodies and certification bodies.
Our commitment towards excellence helped in the pursue and achievement of the international recognition. We have demonstrated to a team of highly qualified professionals our technical competencies for the prestigious standards set for the accreditation scope and our managerial fortes.
Moreover, for us, the pride of being the FIRST Medical Laboratory accredited ISO 15189 in Mauritius is a symbol of “QUALITY, PRECISION, TIMELINESS, RELIABILITY, ACCURACY and CONFIDENTIALITY”; subjecting ourselves to examination by independent bodies. This milestone achieved only strengthened our obsession to conduct medical analyses in an ISO conforming environment and produce quality, reliable and accurate medical results. This is indeed our unique selling proposition, upon which ground we have been challenging competitors’ laboratories around the island since 1993.
Fundamentally, we consider ourselves to be a pride and asset for the growing medical hub in Mauritius as being the pioneer in this accreditation. Biosante Medical Laboratories have a clean and ethical track record for 21 years now and being the FIRST to be accredited ISO 15189 is just another proof of its excellence in the field of medical testing and diagnostic support of the highest quality, with latest technologies.
We invite you to do business with us and in return, along with quality and reliable service we do also promise you a long-term ethical business relationship with 7 days support. We have grown under our partner’s trust.
Our Quality Policy
QUALITY
PRECISION
TIMELINESS
RELIABILITY
ACCURACY
CONFIDENTIALITY
What is ISO 15189:2012 Accreditation?
ISO 15189:2012 specifies requirements for quality and competence in medical laboratories.
ISO 15189:2012 is used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies.
Benefits of ISO 15189 Accreditation
Our laboratories ensure the following:
- Continually improving testing quality and laboratory effectiveness.
- Customer satisfaction achievement through introduction of Quality Management System which is a part of ISO 15189.
- All test equipment is calibrated and traceable to National Standards so that accuracy of results is ascertained & maintained.
- Participation in Inter laboratory Comparison program so that quality level of the lab with respect to other accredited lab can be determined.
- Quality control checks is carried out periodically by which system is always maintained.
- National and international recognition
- Assurance to patients of good laboratory practice
- Meeting purchaser or regulatory specifications
ISO Certificates
Human Resource Quality
ON-THE JOB TRAINING (OFF-THE-JOB Courses)
ON-GOING TRAINING – We believe in constant improvement and life-long learning opportunities. With this view, we support and encourage employees who wish to further develop their professional careers through our many continuing educational programs.
Our staffs have been trained and awarded certificates for the following MQA approved courses in a view to offer better services:
- Customer Service Excellence
- Basic First Aid
- Introduction to Quality Management System
- Team Building
- Internal Audit Principles
- Corporate Governance, Risk and Compliance
- Project Leadership certification
- Team Building, Strategic planning, Vision and Mission development
- Performance management
- Time Management
- Conflict Management
- Professional Grooming and Business Etiquette
- Enhancing productivity through communication
- Statistical Validation Method
Our Analysers
Our analysers are FDA and/ or CE Approved, according to the European Standards
- Biochemistry Analysers
- Haematology Analysers
- Hormones and Electrophoresis Analysers
Quality controls
COMPREHENSIVE QUALITY ASSURANCE PROGRAM – At the heart of our high-performance level is a thorough, responsive Quality Assurance Program (Monitored by our Quality Assurance Manager and Internal Quality Audit function). The system consists of a variety of quality control specimens, both internal and external, comparative review of previous data for consistency and individual, in-depth investigations to identify possible flaws thereby improving our efficiency and accuracy. The program includes horizontal and vertical audits with our referral laboratory partners.
Our Results are validated by a rigorous Internal Quality Control System and by an internationally recognised External Quality Control run by THISTLE (SOUTH AFRICA) and RIQAS (UK) to ensure precise and reliable results.